[News from News Center] Prof. Zhisheng Zhao from Prof. Yongjun Tian’s research team under YSU Metastable Materials Science & Technology SKL, cooperating with scientists at home and abroad, made major progress in studies of carbon materials with previously unachievable property combinations. The research results, entitled“Compressed glassy carbon: An ultrastrong and elastic interpenetrating graphene network”, were published onScience Advanceson June 9th, 2017.Science Advancesis the first comprehensive sub-journal underSciencemagazine.
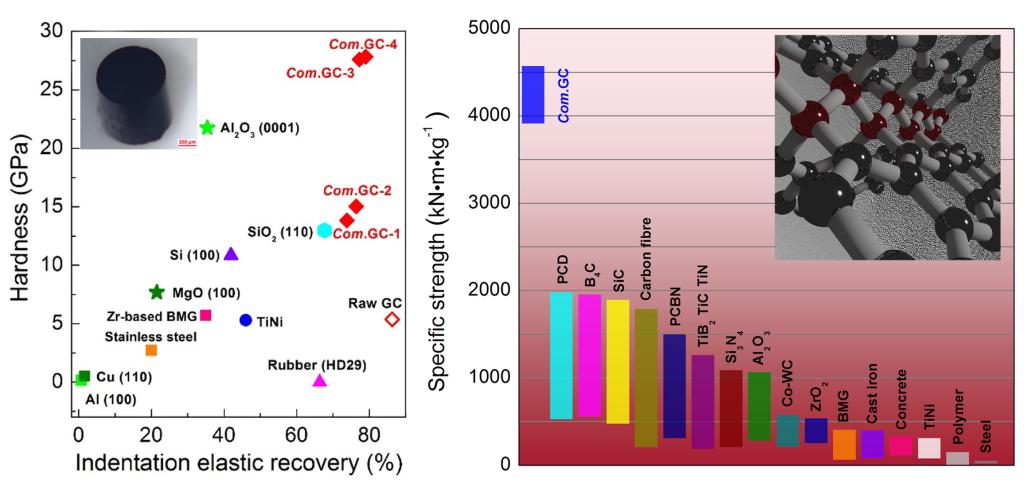
The hardness, elastic recovery and specific strength of Compressed Glassy Carbon (Com. GC)
The left picture is synthetic sample, the right the structure of Com. GC.
There are a lot of allotropes of carbon such as graphite, diamond, carbon nano tube, graphene, and so on. As is known to us, graphite will be converted to diamond under high pressure. The crystal structure of metastable phase got under high temperature and pressure matches closely to the structure of initial precursor, temperature and pressure conditions, and patterns of loading and unloading, which reveals key insights for exploring entirely new carbon materials. In this study, researchers synthesized a new carbon allotrope taking glassy carbon (GC) as raw material under high pressure at moderate temperature condition. It’s named compressed glassy carbon (Com. GC) because it inherits several structural features of GC. Previous researchers found that cold-compressed metastable carbon structure at room temperature would convert back to GC after pressure relief and then the GC would be ultimately transformed to diamond at high-temperature and high-pressure conditions in GC high-pressure research. However, in that research, the pressure and temperature conditions researchers adopted were not enough to support the GC’s conversion to diamond.
Compressed GC, with the bonding features of both graphene and diamond, is a class of new carbon material mixed sp2and sp3. The disordered multilayer graphene in GC curves, bonds and crosslinks together by many means forming a spatial structure with long-range disorder and short-range order. This class of carbon material exhibits extraordinary property combinations with close density and electrical conductivity to graphite, obviously higher compressive strength than common metals and ceramics and even twice higher than commonly used carbon fibers, cemented diamond, silicon carbide and boron carbide ceramics. Its hardness can be compared fairly with gems, thus making it scratch on silicon carbide single crystal. The compressed GC has remarkable above 70% elastic recoveries, also obviously higher than common metals and ceramics, and even higher than the shape-memory TiNi alloy and organic rubber. This class of compressed GC combines a variety of features into one material, including low weight, extraordinary specific compressive strength, high hardness, indentation elastic recovery, and sound electrical conductivity, which has potential applications in military and aerospace fields.
Prof. Lynden A. Archer from Cornell University highlighted this study entitled“From Glassy Carbon to Mixed Carbon”in sectionThis Week in ScienceinSciencemagazine. The essay pointed that“Materials in which these two states (Sp2and Sp3) coexist are highly sought after by materials scientists”and that“Thestudy defines a roadmap for creating bulk carbon-based materials with previously unachievable property combinations”.The news was reported and reposted by dozens of news media at home and abroad, such as Science Alert, Nano Today, IEEE Engineering 360, Carnegie Institution for Science, Wissenschaft aktuel, Russia News Today, Reference News, and so on. Website of National Natural Science Foundation of China also released a report entitled“YSU Made Major Progress in Carbon Materials Studies”.
This study is sponsored by National Natural Science Foundation of China. (Edited by Rui Liu)
Project codes:51672238,51421091,51525205,51332005,51272227
Link to the paper:http://advances.sciencemag.org/content/3/6/e1603213
Link to the report bySciencemagazine:
http://science.sciencemag.org/content/356/6342/twis