Tian Yongjun's team from the High-Pressure Scientific Research Center of YSU, in collaboration with domestic and international researchers, has theoretically predicted that under pressure, the noble gas helium (He) can react with fluorine (F2) to form a polar covalent He–F bond, resulting in a stable compound He3F2. The research findings, titled "Chemical Bonding between Helium and Fluorine under Pressure", were published in the Journal of the American Chemical Society.
In the world of chemical elements, fluorine is known for its status as the most electronegative element in the universe, while helium stubbornly maintains its chemical inertness, refusing to react with any other element under normal conditions. Yet, researchers of YSU have made this "ice and fire" elemental pair react under extreme conditions, forming a true helium bond for the first time—overturning conventional scientific understanding.
Through theoretical calculations, the researchers found that under extreme pressure of 2 TPa, fluorine atoms overcame helium's chemical inertness, resulting in the formation of a He3F2 compound composed of herringbone HeF2 chain-like structural units and isolated helium atoms.
3He + F2 → He3F2 (i.e., HeF2‧2He)
Chemical bonding analysis reveals that the 1s electrons of helium in He3F2 participate in bond formation, creating a novel polar covalent He-F bond. With each helium atom bonded to three fluorine atoms, each fluorine atom connected to two helium atoms, and charge transfer between the two elements, this bonding pattern gives rise to herringbone HeF2 chain-like structural units. Molecular orbital theory analysis further confirms that the polar covalent He–F bond arises from strong interactions between the 1s orbital of helium and the 2p orbitals of fluorine under pressure. This type of bonding, typically observed in molecules like water, has now been achieved between the two most unlikely elements. This finding not only pushes the boundaries of fluorine's reactivity, but also unveils the hidden chemical potential of inert elements under extreme conditions, providing valuable insights into the discovery of new substances and reactions in such environments. The research has been highly praised by reviewers. For example: "This is an important contribution in the field of noble gas binding, formation of a helium bond under pressure is interesting." Another reviewer commented: "This is in contrast to other studies of He-containing high-pressure materials, where the He tends to act as an inert stabilizing spacer…This certainly is very novel chemistry."
This research was jointly conducted by YSU, Nankai University, and University of North Carolina. The co-first authors of the paper are Dr. Hou Jingyu, a postdoctoral researcher of the School of Materials Science and Engineering, YSU, and Wang Xiaojun, a doctoral student of the School of Science, YSU. The corresponding authors are Professor Zhou Xiangfeng from YSU and Professor Dong Xiao from Nankai University. This research was funded by the National Natural Science Foundation of China (Grant Nos. 52025026, 52288102, 52090020, 92263101, 12174200), among other funding programs.
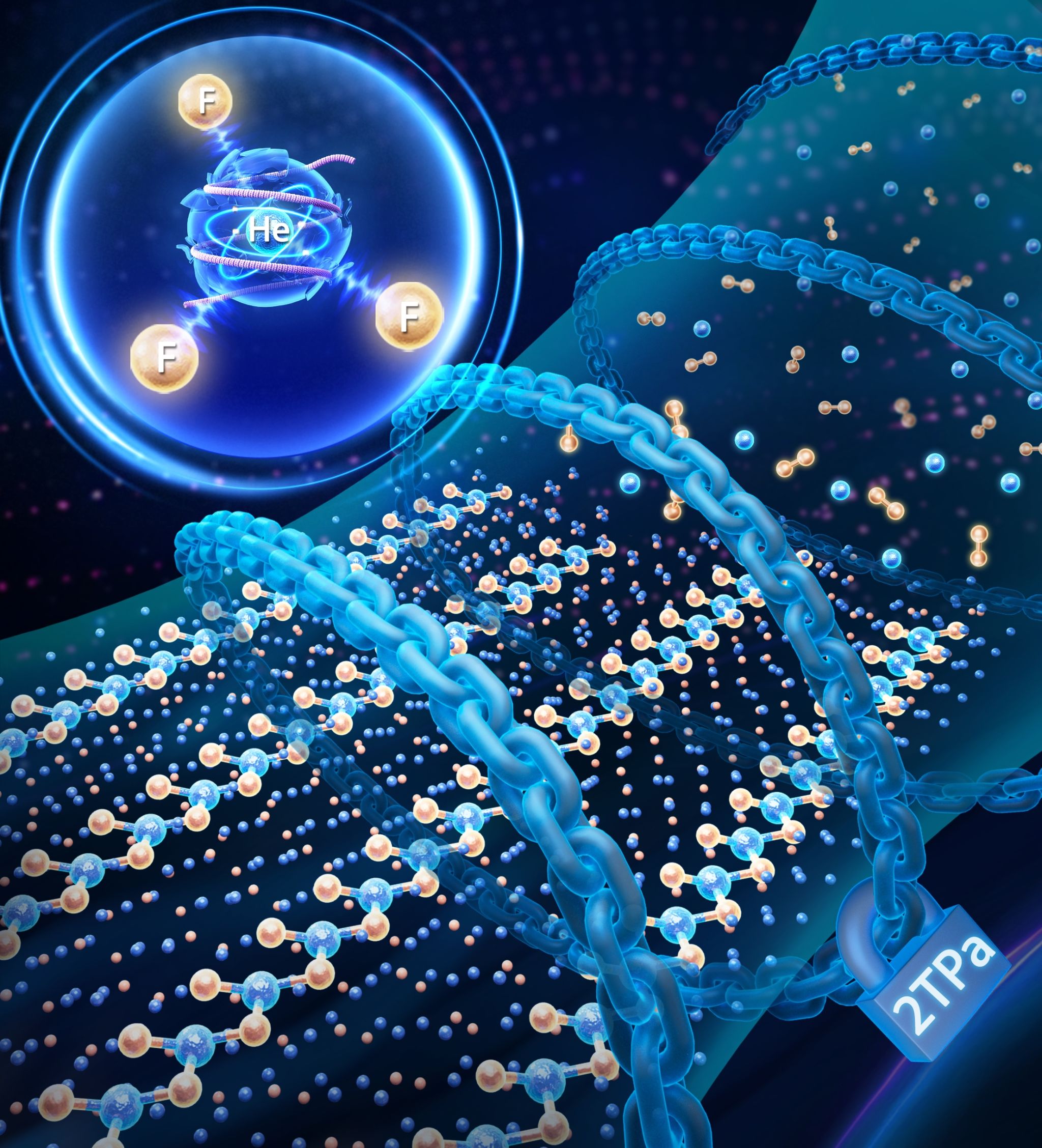
Chemical bonding between helium and fluorine under pressure