Researchers at YSU, led by Professors Gao Faming and Hou Li, have developed a nickel (oxy)hydroxide catalyst co-engineered with iron and oxygen vacancies to boost the performance of the oxygen evolution reaction (OER). The advance enables synergistic coupling between the adsorbate evolution mechanism (AEM) and the lattice oxygen mechanism (LOM), overcoming the long-standing challenge of balancing catalytic activity and stability. The findings, published in Nature Communications on October 2, 2025 (Nat Commun 16, 8788 (2025)), are presented in a paper titled “Iron and Oxygen Vacancies Co-Modulated Adsorption Evolution and Lattice Oxygen Dual-Path Mechanism for Water Oxidation”.
Alkaline water electrolysis is emerging as one of the most promising technologies for green hydrogen production, but its efficiency remains limited by the sluggish four-electron OER at the anode. The challenge becomes even greater in seawater, where insoluble impurities and competing chlorine oxidation reactions (ClOR) raise the overpotential and accelerate electrode degradation. The performance of OER catalysts depends largely on the reaction pathways at their active sites. The AEM on metal centers is restricted by a linear scaling relationship between OH and OOH intermediates, leading to a high theoretical overpotential. In contrast, the LOM on oxygen ligands reduces the energy barrier through direct O-O coupling but compromises structural stability as lattice oxygen atoms are repeatedly lost and replenished. Put simply, the AEM route is sturdy but slow, whereas the LOM route is faster but less stable. Activating both pathways simultaneously has thus become a key strategy for developing next-generation OER catalysts that combine high activity with long-term durability for industrial-scale water electrolysis.
In their latest study, researchers at YSU have developed an Fe-Ni₂P/NiMoO₄ composite precatalyst that reconstructs during electrochemical activation into an iron- and oxygen-vacancy co-modulated γ-NiOOH (OV-Ni(Fe)OOH) active phase. The coordinated tuning of Fe and oxygen vacancies adjusts the Ni-O bonds into an “optimal covalency window”, enabling both the AEM on Ni sites and the LOM on O sites to work synergistically for enhanced oxygen evolution. Using advanced in-situ techniques, including isotope-labeled differential electrochemical mass spectrometry and surface-enhanced infrared absorption spectroscopy, alongside density functional theory (DFT) simulations, the researchers demonstrate that oxygen vacancies accelerate AEM kinetics, while Fe incorporation increases lattice oxygen activity, achieving strong AEM-LOM coupling within a single active phase. Additional analyses, including constant-potential projected density of states (PDOS) mapping and ab initio molecular dynamics simulations, reveal that moderate oxygen vacancies at high potential enhance intermediate adsorption on Ni sites and suppress excessive lattice oxygen activation, thereby improving catalyst stability without compromising OER performance. The study offers atomic-level insight into how Fe and oxygen vacancies reshape the electronic structure of Ni and O sites and provides a theoretical foundation for designing next-generation dual-pathway catalysts with high activity and long-term durability.
In performance tests, the YSU-developed catalyst demonstrates exceptional efficiency in both freshwater and seawater electrolysis. Owing to the coexistence of AEM and LOM pathways within a single active phase, it achieves industrial-level current densities of 1.0 A cm⁻² at overpotentials of only 274.5 mV in alkaline freshwater and 299.1 mV in alkaline seawater. The catalyst also shows remarkable durability, maintaining stable operation for 1,000 hours in freshwater and 500 hours in seawater. When integrated into an anion-exchange membrane electrolyzer, it sustains continuous seawater electrolysis for 500 hours at 1.0 A cm⁻² with no significant degradation. This breakthrough highlights strong potential for industrial-scale water electrolysis and hydrogen production. Beyond setting new performance benchmarks, the study introduces a “dual-mechanism coupling” design paradigm for non-precious-metal catalysts and provides critical support for advancing China’s green hydrogen development toward direct seawater utilization and long-lifetime operation.
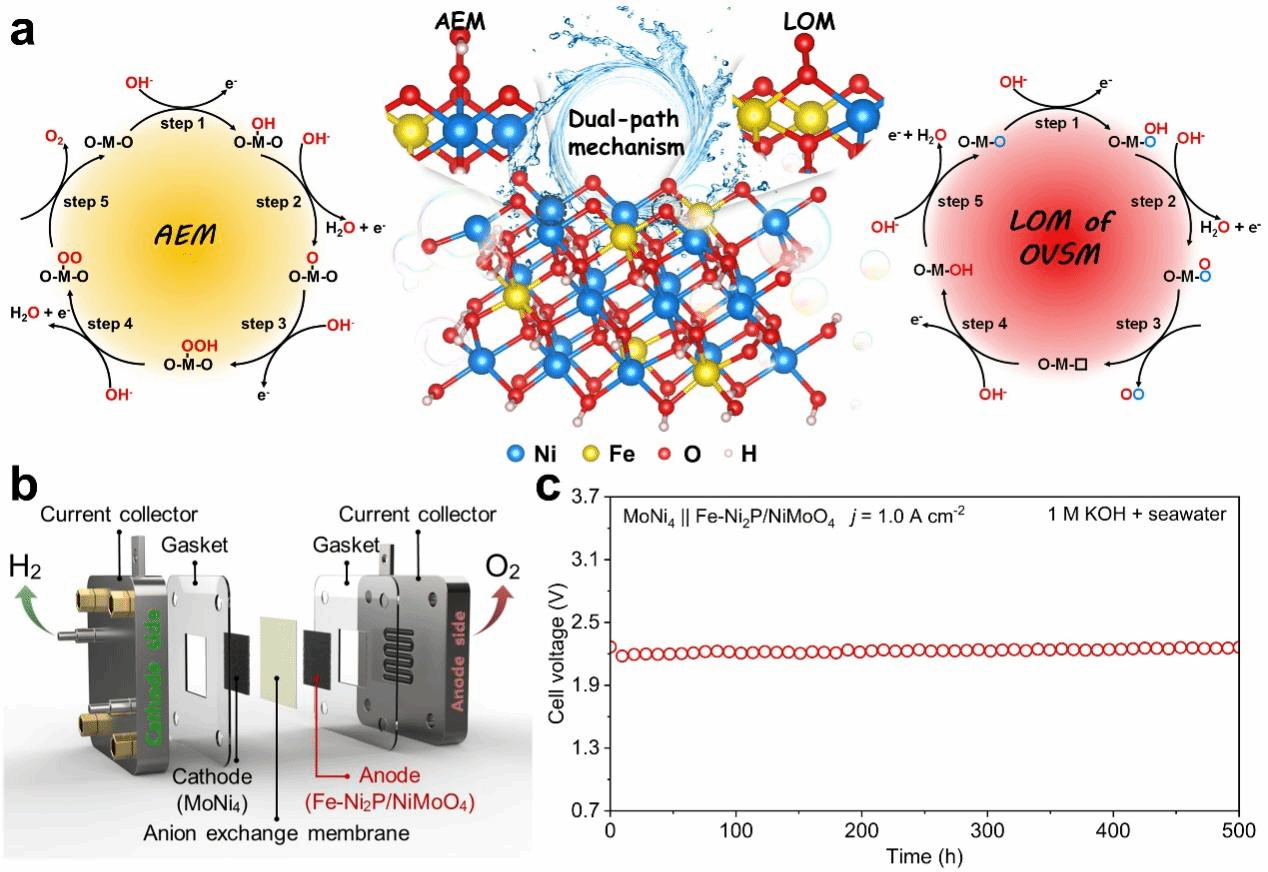
Illustration of AEM-LOM coupling pathways and corresponding anion-exchange membrane electrolyzer performance
The research was jointly funded by the National Natural Science Foundation of China (Grant No. 21371149) and the Natural Science Foundation of Hebei Province (Grant No. B2021203016). The paper names YSU doctoral student Tao Xiwen as first author, with Professors Hou Li and Gao Faming serving as corresponding authors.