The research team led by Academician Tian Yongjun from the State Key Laboratory of Metastable Materials Science and Technology at YSU, in collaboration with researchers from the University of Science and Technology of China, Zhejiang University, Zhejiang University of Technology, and Iowa State University (USA), has successfully demonstrated that the ceramic crystal boron carbide (B4C) exhibits room-temperature ductility comparable to metals. This groundbreaking discovery challenges the conventional understanding of the brittleness of covalent materials and provides a new research direction for enhancing the ductility of strong covalent materials. The related findings were published in Science Advances (Volume 11, Page 4648) on April 9, 2025.
Ductility refers to a material’s capacity for substantial plastic deformation before fracture. This property is of paramount importance for engineering applications and resistance to mechanical overload. However, unlike metallic materials that achieve plasticity through dislocation glide and twinning mechanisms, covalent materials typically exhibit extreme brittleness at room temperature due to their strong, directional covalent bonds and lack of slip systems. B4C, as a typical covalent material, is widely used in ballistic armor and engineering applications due to its exceptional hardness, low density, and high melting point. However, its inherent brittleness has severely restricted its use in scenarios where ductility and toughness are required. Although previous studies suggested that B4C might exhibit localized plastic deformation under specific conditions (such as compression or impact), achieving significant tensile plastic deformation at room temperature for the material remained an unresolved scientific challenge.
In this study, through state-of-the-art four-dimensional scanning transmission electron microscopy (4D-STEM) and atomic simulations, the research team revealed a novel mechanism for achieving high ductility in B₄C at room temperature. Using electron-optical imaging techniques, the researchers clearly observed the presence of “carbon-vacancy-carbon” chains in B₄C crystals (Figure 1). The vacancies caused by missing boron atoms in these chains significantly altered the local lattice structure. Atomic simulations showed that these vacancies could induce the formation of carbon-carbon bonds, leading to local lattice disorder (local amorphization) and enabling plastic deformation. During in situ tensile testing of micro/nano B4C samples, the research team observed that the samples exhibited a fracture strain of up to 31.2% and ductility of 26.8% after exceeding the elastic limit (Figure 2). These values far surpassed previous expectations for covalent materials and even approached the ductility levels of some metallic materials. Furthermore, in situ high-resolution transmission electron microscopy (HRTEM) observations revealed that localized regions of the B₄C crystal underwent amorphization during stretching, forming 1–2 nm wide amorphous domains. These domains expanded and interconnected, ultimately forming amorphous bands that became the primary contributors to plastic deformation. The study also demonstrated that as the sample size decreased (below 100 nm in thickness), the fracture strain and strength of B4C increased significantly, indicating that size plays a significant role in the plastic deformation of B4C.
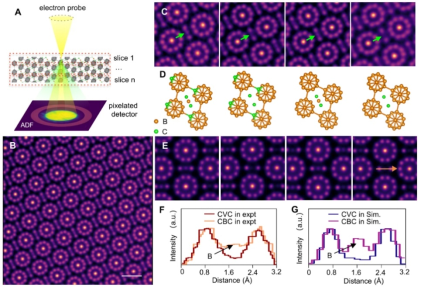
Figure 1: Precise characterization of atomic structure and boron vacancies in B4C crystals via 4D-STEM imaging
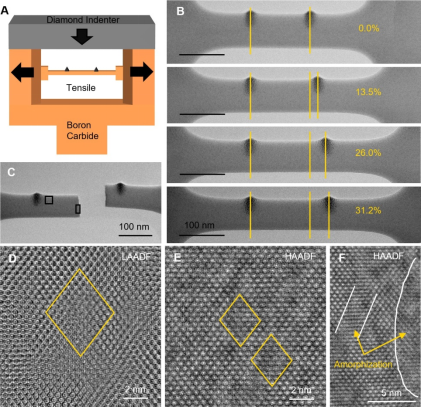
Figure 2: In situ tensile deformation process of B4C nanobeams and high-resolution STEM characterization of the plastic deformation zones
This study not only reveals the critical role of boron vacancies in plastic deformation but also proposes a new material design strategy: introducing specific lattice vacancies can significantly improve the ductility of strong covalent materials. The findings suggest great potential for expanding B4C’s applications, as enhanced ductility could prove to be of considerable benefit in scenarios where high toughness and impact resistance are required, such as in the aerospace and protective armor industries. Moreover, the “vacancy-induced amorphization” mechanism discovered in the study may also apply to other strong covalent materials (e.g., boron nitride, silicon carbide), providing valuable theoretical guidance for improving their plasticity and toughness. This breakthrough discovery offers a new perspective for the design and application of strong covalent materials.
The study was conducted through the collaboration between YSU and several universities and colleges from China and the USA. The co-first authors of the paper are Li Penghui, Li Jun, and Feng Qilong, while the corresponding authors are Jin Tianye, Ning Shouzong, An Qi, and Nie Anmin. The project is funded by the National Natural Science Foundation of China (52288102, 52090022), the Natural Science Foundation of Hebei Province (E2024203054, E2022203109), and other programs.