The research group led by Professor Yang Guochun from the School of Science at YSU has, through theoretical research, proposed a pressure-stable ternary semiconductor electron compound, Li10AuF. This structure achieves a negative heptavalent state for gold (Au) based on a lithium-fluorine polarization combined pressure-regulated orbital energy level mechanism, while enabling stable coexistence with paired interstitial anionic electrons. Recently, the research findings were published online in Nature Communications under the title "Stabilization of Gold in the Negative Heptavalent State Driven by Lithium-Fluorine Polarization Under High Pressure".
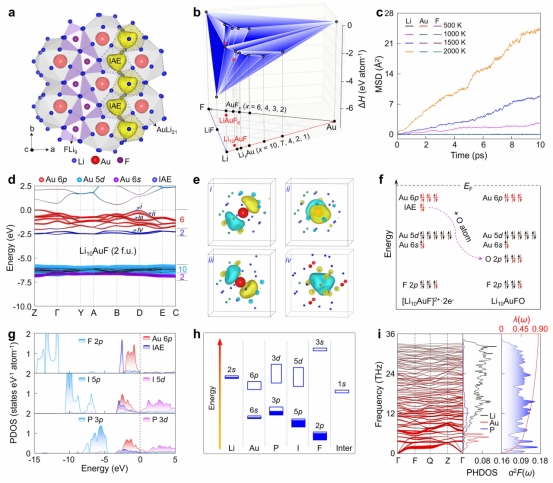
Crystal Structure, Interstitial Electrons, Stability, Electronic Structure, Oxidation State Regulation Mechanism, and Evolution of Electronic Properties Modulated by X Elements of Li10AuF
Exploring unconventional oxidation states represents an important frontier direction in the fields of chemistry and physical sciences, holding significant implications for expanding elemental bonding patterns and revealing material electronic structure mechanisms. The research group recently proposed an innovative "dual-driven" strategy: under high-pressure conditions, the strong reducing agent lithium (Li) and the strong oxidizing agent fluorine (F) are synergistically introduced, achieving a negative heptavalent state for gold (Au) in the ternary electron compound Li10AuF. This research breaks through the traditional paradigm of oxidation state regulation, offering new insights into exploring abnormal chemical valences of elements under extreme conditions.
The research found that this insulating phase not only stabilizes the extreme negative oxidation state of Au but also forms paired localized anionic electrons within the crystal lattice interstices, causing the Au atom to exhibit an electronic configuration equivalent to that of radon (Rn). This challenges the traditional understanding that interstitial anionic electrons are difficult to coexist with highly reduced metal centers in the same compound, providing a new perspective for understanding and regulating electron correlations in extreme chemical valence systems.
Further research indicates that by replacing F with iodine (I) or phosphorus (P), the negative charge on Au and the localization degree of interstitial electrons can be gradually weakened, transforming the insulating state of Li10AuF into a semimetallic state of Li10AuI, ultimately leading to superconducting behavior in Li10AuP. These results reveal the synergistic mechanism between chemically polarized element combinations and high-pressure conditions in regulating extreme oxidation states and electron distributions.
This research was supported by multiple projects, including the National Natural Science Foundation of China, the Beijing National Laboratory for Condensed Matter Physics, and the Natural Science Foundation of Hebei Province. The first author of the paper is Zhang Xiaohua from the School of Science, and the corresponding author is Professor Yang Guochun from the School of Science.
The research group has long focused on theoretical studies of pressure-induced new chemistry and the superconductivity of electron compounds, and has published several important research findings in internationally renowned journals such as Journal of the American Chemical Society, Angewandte Chemie, and Physical Review Letters, making positive contributions to advancing the field.