The research team led by Professor Huang Jianyu from the State Key Laboratory of Metastable Materials Science and Technology at YSU, in collaboration with the research team from the Institute of Physics, Chinese Academy of Sciences (IOP, CAS), and Yangtze River Delta Physics Research Center Co., Ltd., systematically decoupled the interactions between different gases and Na-layered oxides using a combination of advanced characterization methods, elucidated the degradation pathways, developed innovative quantitative methods to compare the air sensitivity of different materials, identified the underlying dominant factors and proposed rational modification design principles. The research findings were published in Science on August 15, 2024 [Science Vol. 385, No. 6710] as a paper titled “Decoupling the air sensitivity of Na-layered oxides”. Link: https://www.science.org/doi/10.1126/science.adm9223.
Na-layered oxides, featuring excellent high capacity and scalable production, play a crucial role in the field of lithium-ion and sodium-ion batteries. Thanks to the abundant availability of sodium resources and the high flexibility in the selection of transition metal elements - without the need to rely on expensive cobalt and nickel, but using more cost-effective iron and copper as substitutes - Na-layered oxides exhibit significant cost advantages. However, the air sensitivity of these materials has plagued the research community on Na-layered oxides for over four decades, posing a significant hurdle to their commercialization.
The research team pointed out that breaking the coupling between gases is a key external factor for achieving stable material storage. By using the widely studied NaNi1/3Fe1/3Mn1/3O2 (NFM111) as a model material and extending to its homologs, combined with the use of advanced characterization methods such as in-situ environmental atmosphere transmission electron microscopy, isotopic labeling, secondary ion mass spectrometry, neutron scattering, and synchrotron X-ray absorption spectroscopy, the team found that water vapor, carbon dioxide, or oxygen alone does not trigger significant degradation reactions, which challenges the conventional wisdom that these three gases (especially water vapor) alone can trigger severe degradation reactions. Water vapor plays a critical bridging role in the degradation process by connecting carbon dioxide and oxygen with the material to trigger acidic degradation and oxidative degradation respectively. Acidic degradation will lead to intense Na+/H+ exchange, forming sodium carbonate or sodium bicarbonate on the material surface, as well as subsequent reactions such as crack propagation, lattice distortion, dislocation generation, and reduction and reconstruction of surface transition metal ions under strongly acidic conditions. In oxidative degradation, transition metal ions with lower oxide redox potentials (closer to the Fermi level) in the bulk phase will be preferentially oxidized, releasing sodium ions to the surface to balance the charge. The oxidized transition metal ions (Ni3+) are usually unstable on the surface and easily reduced, leading to surface reconstruction.
The research team also developed a standardized air sensitivity testing method based on titration gas chromatography technology to quantitatively evaluate the contributions of different reaction pathways and the air sensitivity of different materials. Based on the quantitative results of sodium loss in over 30 degraded materials and previous research findings, and combining the ionic potential and initial sodium content of each component, the team defined the cation competition coefficient η and found that acidic degradation dominates the degradation reaction of most materials. Reducing the cation competition coefficient and increasing the material particle size can effectively improve the material’s acid resistance, and selecting high-potential redox pairs can effectively improve the material’s oxidation resistance. By combining the above methods and introducing potential surface and alkali metal layer optimization, the sodium loss of the modified material Na0.96Ca0.02Cu0.1Ni0.35Fe0.1Mn0.2Ti0.25O2 can be reduced to 0.019 from 0.489 of the model material (a 96% reduction). The research findings have provided important guidance for the design of air-stable layered oxides for sodium-ion batteries.
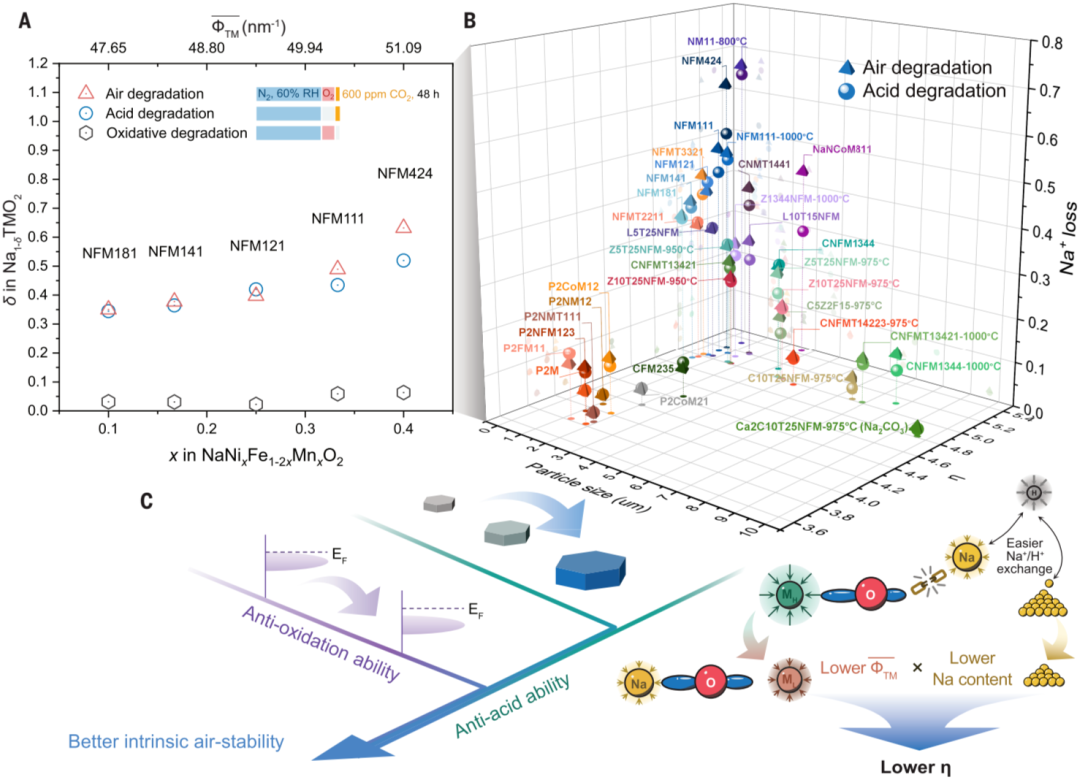
Figure: quantification of acidic and oxidative degradation and the design principles for intrinsically air-stable sodium-ion layered oxides
The research was funded by the Natural Science Foundation of Hebei Province (B2024203054). Yang Yang, a doctoral student from IOP, CAS, and Wang Zaifa, a doctoral graduate from YSU, are the first authors of this paper.