[News from the News Center] Recently, YSU research team consisting of four associate professors, including Fan Yuqian, Huang Weiwei, Ma Zhipeng and Guo Wenfeng, from the School of Environmental and Chemical Engineering made an original breakthrough in the research of anode materials based on alkaline system energy storage, and proposed a kind of based on the anode energy storage mechanism of Co2+/Co0 redox reaction, the key problems of anode energy storage are well solved. The research result is published withEnergy Storage Materialstitled as Co(OH)2@Co electrode for efficient alkaline anode based on Co2+/Co0redox mechanism (impact factor: 13.1). YSU is the first institution, Fan Yuqian is the first author, co-authored with corresponding authors YSU Guo Wenfeng and Prof Mai Liqiang from Wuhan University of Technology.
Research on energy storage based on alkaline systems has attracted great interest and has made great progress, especially in the research of super-capacitor materials. Previous studies have shown that some transition metal (TM) materials (such as products based on Co, Ni and Mn) exhibit excellent performance in alkaline systems. Most of the research results show that these TM materials for alkaline energy storage can achieve higher rate performance, longer life and better safety than existing lithium-ion batteries and lead-acid batteries. Therefore, the development of new energy storage materials based on alkaline systems or the proposed new energy storage mechanism is beneficial to the development of next-generation RBs. However, it is unfortunate that TM products currently in hot research, such as Co-, Ni-, Mn-oxide/hydroxide/sulphide, have relatively high operating potentials in alkaline systems (> 0 V vs Ag / AgCl), so they can only be used as cathode materials.
In contrast, the development and research of anode materials (working potential: <0V vs ag agcl) under alkaline system is very scarce, and related reports are rare. at present, although some sporadic tm products such as activated carbon, graphene-related products, carbon-based materials, and fe-oxide/hydroxide, vn, moo3, etc. have been reported as anode materials, compared with existing cathode materials, the anode materials reported so far are still much inferior in terms of energy storage capacity and high rate performance, and the anode problem needs to be solved urgently.
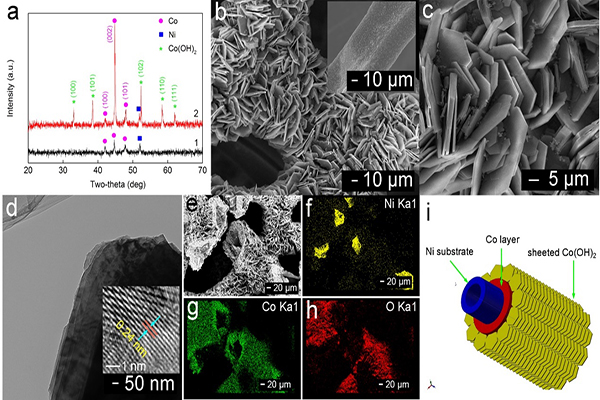
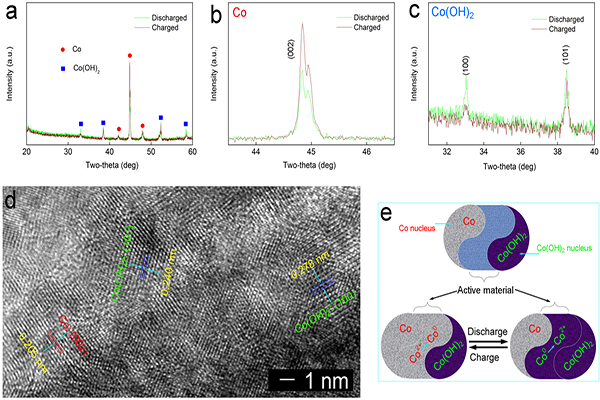
In the research reported, a Co(OH)2@CoF electrode was obtained by preparing a sheet of Co(OH) 2 array in situ on a foam Co (CoF). The physical characterization shows that the Co(OH)2 film prepared in situ is composed of a separate hexagonal sheet structure, and the energy storage mechanism of the electrode based on Co2+/Co0 in the alkaline system is proved. As a thin film electrode without the use of a binder (Binder-free), the Co(OH)2@ CoF electrode achieves an extremely high area capacity (19.26-17.15 mAh cm-2, current 50-250 mA cm-2) and good circulation and stability (1500 times, 100 mA cm-2). The assembled cell has an energy density of 198.3-132.9 Wh m-2 (corresponding power density: 539.5-2355 W m-2), exhibiting excellent surface energy density and rate performance. This work was supported by the China Postdoctoral Science Foundation Project (No.2015M581314), the Hebei Natural Science Foundation Youth Fund (No. B2015203350) and the Yanshan University Research Fund (No. 14LGA016).
Related Figures
[Translated by Ni Rongzi]