[News from the News Center]Recently,the research team led byProf.Han Shumin and Prof.Wang Limin of ouruniveristycooperated with Prof.Ji Xiului of the Oregon State University in the United States to design a new Zn//V2O5 battery supported by ZnCl2 water-soluble salt for the first time by utilizing the high solubility characteristics of ZnCl2 in water, which producesZB with high capacity and long cycle life. Relevant research results were recently published in the internationally important journal “Advanced functional materials”.
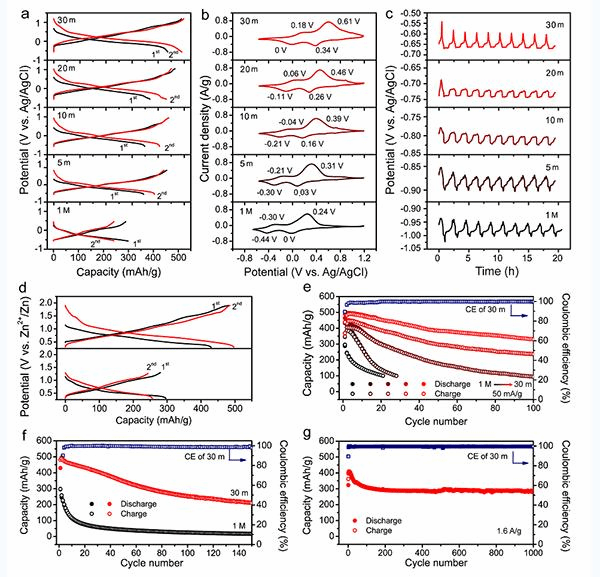
It is found that the increase of the concentration of ZnCl2 water-soluble salt electrolyte can effectively improve the working voltage of the V-based metal oxide of the positive electrode material, broaden the electrochemical window and inhibit the dissolution of the positive electrode material, and significantly improve its electrochemical performance. When the concentration of ZnCl2 electrolyte increases from 1 M to 30m, the discharge capacity of the V-based metal oxide of the positive electrode material increases from 296 mAh g–1 to 496 mAh g–1, and the absolute potential increases by 0.4 V; in particular, at 50 mA. When the g–1 charge/discharge cycle is performed at a small current density, the 100-week capacity retention rate is increased by more than 6 times.
The rapid development of new energy vehicles and renewable energy power storage technology has placed an urgent need for high-security, low-cost electrochemical energy storage systems (batteries). Compared with organic solution electrolytes, aqueous electrolytes do not have the risk of burningbuthave the advantages of low price, no pollution, high power density, etc., and have broad application prospects in the field of large-scale energy storage. Among various water-based metal ion batteries, water-based zinc batteries (ZB) are considered to be one of the most practical new types of chemical power sources due to their high energy density, low cost and high safety. ZB uses zinc metal as the negative electrode, and cation-deficient material as the positive electrode, mainly including Mn-based or V-based metal oxide, Prussian blue analog and organic positive electrode material, and a near-neutral or weakly acidic aqueous solution containing Zn2+ as an electrolyte. At present, the main problem faced by the commercial application of ZB batteries is the low efficiency of the deposition/precipitation of the negative zinc metal during the electrochemical cycle and the rapid decay of the capacity caused by the dissolution of the active material of the positive electrode material.


The researchfinding is coauthored: Zhang Lu thepost of postdoctoral researcheras the first author, Prof. Han Shumin, Prof. Wang Limin, and Prof. Ji Xiulei together as corresponding author.
[Translated by Yu Miao]